Overall Equipment Effectiveness (OEE) is a critical metric in pharmaceutical manufacturing, ensuring optimal production efficiency while maintaining strict compliance with industry regulations. OEE helps manufacturers measure and improve equipment performance by assessing availability, performance, and quality. By implementing OEE strategies, pharmaceutical companies can minimize downtime, reduce waste, and enhance product quality.
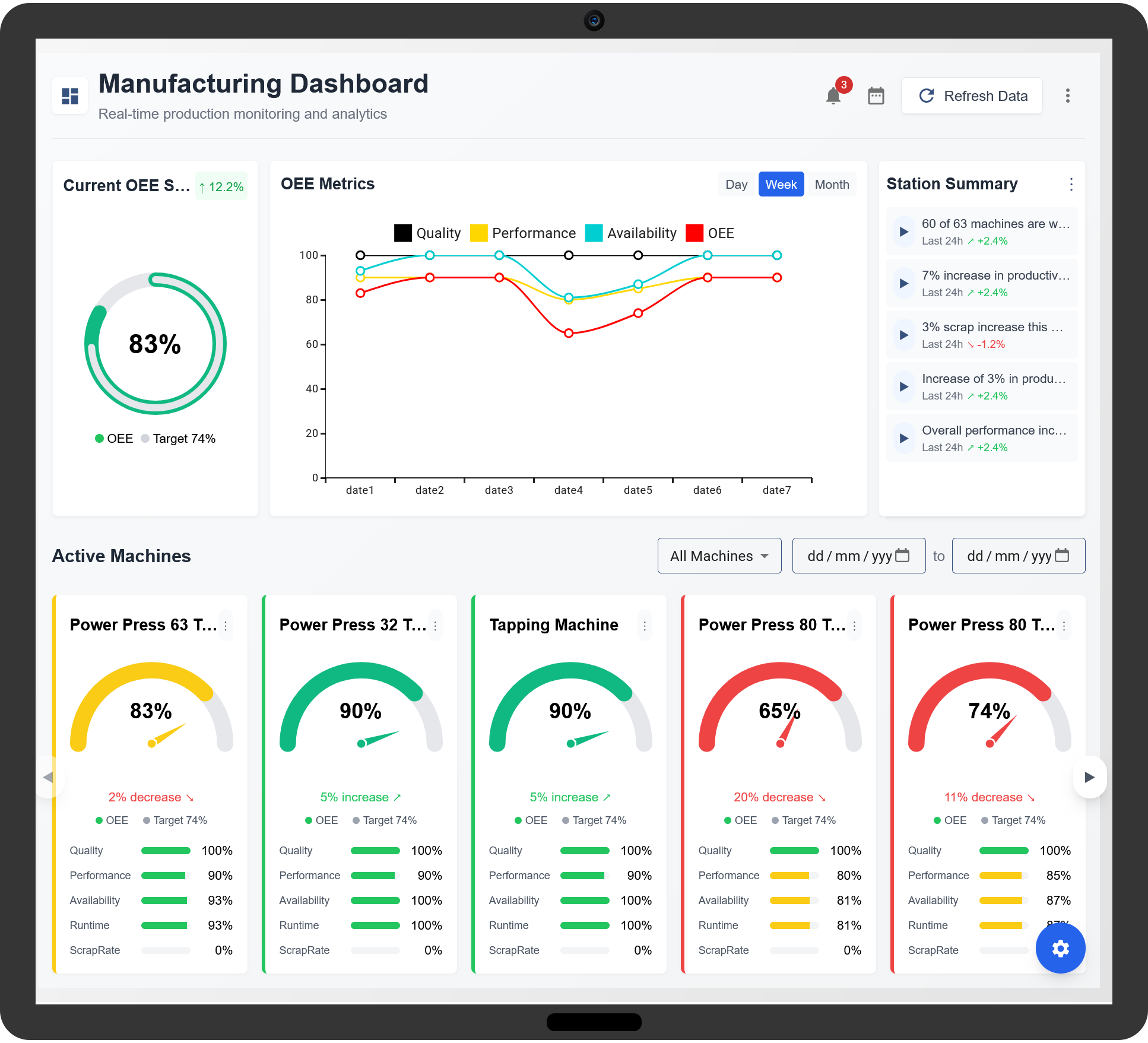
Understanding OEE in Pharmaceutical Manufacturing
OEE is calculated using three key factors:
- Availability: Measures the operational time of equipment versus planned production time. Equipment failures, maintenance, and changeovers impact availability.
- Performance: Evaluates whether machines operate at optimal speeds. Slow cycle times and minor stoppages reduce performance.
- Quality: Assesses the ratio of defect-free products to total production output. Deviations, contamination, or incorrect formulations lower the quality score.
Multiplying these three factors provides the overall OEE score, which highlights production inefficiencies and areas for improvement.
Challenges in Pharmaceutical Manufacturing
Pharmaceutical production faces unique challenges that impact efficiency and compliance, including:
- Strict Regulatory Requirements: Compliance with manufacturing standards requires thorough documentation and validation, affecting production speed.
- Complex Batch Processing: Pharmaceutical products require precise formulations and quality checks, increasing process complexity.
- Frequent Changeovers: Cleaning and reconfiguring equipment between batches lead to significant downtime.
- High Cost of Downtime: Equipment failures or production delays result in financial losses and supply chain disruptions.
- Product Quality and Safety: Any deviation in formulation or contamination risks product recalls and compliance violations.
Implementing OEE in pharmaceutical manufacturing helps address these challenges by optimizing equipment utilization and maintaining high-quality standards.
How OEE Improves Pharmaceutical Manufacturing
1. Reducing Equipment Downtime
Unplanned downtime can disrupt pharmaceutical production and lead to compliance issues. OEE monitoring provides real-time data on machine performance and predictive maintenance insights, allowing manufacturers to schedule maintenance before breakdowns occur.
2. Optimizing Batch Changeovers
Frequent changeovers are necessary in pharmaceutical manufacturing due to different formulations and regulatory requirements. OEE analysis helps identify inefficiencies in cleaning and setup procedures, reducing downtime while ensuring compliance.
3. Enhancing Production Line Efficiency
Monitoring cycle times and tracking minor stoppages help optimize machine performance. Adjusting process parameters, streamlining workflows, and automating manual tasks improve overall production efficiency.
4. Ensuring High Product Quality
Quality control is essential in pharmaceutical manufacturing. OEE software tracks defect rates, deviations, and batch inconsistencies, helping manufacturers implement corrective actions to reduce rework and waste.
5. Maintaining Compliance and Traceability
Regulatory agencies require detailed production records and traceability of pharmaceutical products. OEE solutions integrate with tracking systems to provide accurate data, ensuring compliance with manufacturing standards.
Key Features of OEE Implementation
- Real-Time Monitoring: Provides visibility into production performance and equipment status.
- Automated Data Collection: Reduces manual errors and improves compliance reporting.
- Predictive Maintenance: Prevents unexpected breakdowns by analyzing equipment trends.
- Compliance Tracking: Ensures adherence to pharmaceutical manufacturing regulations.
- Performance Benchmarking: Compares efficiency metrics with industry best practices.
- Customizable Dashboards: Displays key production metrics for informed decision-making.
Steps to Implement OEE in Pharmaceutical Manufacturing
1. Define Performance Metrics
Establish OEE benchmarks for availability, performance, and quality. These metrics help manufacturers identify inefficiencies and improve production processes.
2. Implement OEE Monitoring Tools
Deploy software solutions to collect real-time production data, track machine performance, and generate reports. Automated data collection minimizes errors and ensures compliance.
3. Analyze Production Data
Review OEE reports to identify common causes of downtime, speed losses, and quality defects. Using data-driven insights, manufacturers can implement corrective measures to enhance efficiency.
4. Optimize Equipment Performance
Fine-tune machine settings, adjust batch processing speeds, and integrate automation solutions to improve overall production efficiency.
5. Continuous Improvement
Regularly review OEE metrics, conduct performance assessments, and implement best practices to sustain long-term efficiency improvements.
OEE in Pharmaceutical Manufacturing
What is OEE in pharmaceutical manufacturing?
OEE (Overall Equipment Effectiveness) measures the efficiency of production processes in pharmaceutical manufacturing by evaluating equipment performance, availability, and quality output.
Why is OEE important in pharmaceutical manufacturing?
OEE is essential in pharmaceutical manufacturing to ensure consistent production, minimize waste, optimize resources, and comply with strict regulatory standards.
How does OEE contribute to improving production efficiency in pharmaceuticals?
By identifying and addressing inefficiencies, OEE enhances production flow, reduces downtime, and ensures that manufacturing processes are operating at their maximum potential.
What are the key components of OEE in pharmaceutical production?
The three main components of OEE are availability (equipment uptime), performance (speed of production), and quality (rate of defect-free products).
How can OEE help reduce downtime in pharmaceutical manufacturing?
OEE tracks downtime causes, enabling manufacturers to identify common issues such as equipment failures or process inefficiencies and address them proactively.
How does OEE support compliance with pharmaceutical industry regulations?
OEE ensures that pharmaceutical manufacturers are consistently following regulations by maintaining high-quality standards, performing regular equipment maintenance, and keeping track of manufacturing processes.
How does OEE contribute to quality control in pharmaceutical manufacturing?
OEE helps ensure quality by tracking defect rates, reducing variations in production, and maintaining strict control over processes to meet regulatory standards.
What challenges affect OEE implementation in pharmaceutical manufacturing?
Challenges include managing complex manufacturing processes, ensuring regulatory compliance, handling variability in raw materials, and dealing with the need for frequent changeovers.
How does OEE enhance overall production reliability in pharmaceutical manufacturing?
By continuously monitoring equipment performance and quality, OEE helps identify bottlenecks and inefficiencies, improving the reliability of the entire production process.
Can OEE help in minimizing production costs in pharmaceutical manufacturing?
Yes, OEE can minimize production costs by improving equipment uptime, reducing waste, and enhancing overall operational efficiency.
How does OEE data help pharmaceutical manufacturers improve equipment maintenance?
OEE data reveals patterns in equipment performance, allowing manufacturers to schedule preventive maintenance and address potential issues before they lead to breakdowns.
How does OEE improve batch consistency in pharmaceutical production?
By improving process control and reducing downtime, OEE helps ensure that each production batch is consistent in terms of quality and output.
How does automation impact OEE in pharmaceutical manufacturing?
Automation increases OEE by enhancing production speed, reducing human errors, and ensuring more consistent quality, which are crucial in the pharmaceutical industry.
What is the relationship between OEE and supply chain management in pharmaceuticals?
OEE impacts supply chain efficiency by ensuring that production equipment is reliable and performing optimally, thereby supporting timely product delivery and reducing supply chain disruptions.
How can OEE be continuously improved in pharmaceutical manufacturing?
OEE can be improved through regular performance analysis, continuous training for operators, equipment upgrades, and implementing lean manufacturing principles to address inefficiencies.